产品说明
一般描述
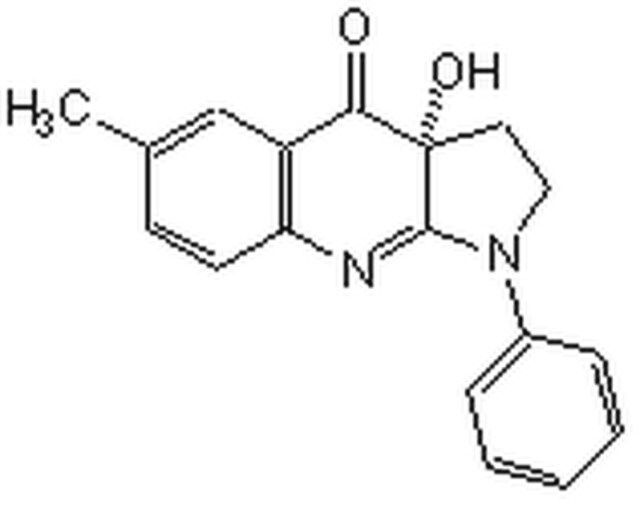
A cell-permeable racemic mixture of Halofuginone, a halogenated derivative of the Dichroa febriguga alkaloid Febrifugine, whose D-(+)/(2R, 3S) enantiomer serves as the active inhibitor against ProRS- (prolyl-tRNA synthetase) mediated aminoacylation by simultaneously targeting/blocking ProRS Proline-binding pocket with its hydroxypiperidine and tRNA 3′ end adenosine-binding site with its halogenated 4-quinazolinone, preventing not only ProRS-catalyzed prolyl adenylation (Pro-AMP formation), but also the subsequent Pro transfer from Pro-AMP to tRNAPro. Consistently, only proline, but not other NEAA, is able to reverse the inhibitory effect of HF in in vitro rabbit reticulocyte lysate translations (1 µM HF; 1 mM Pro) and prevent HF-induced cellular AAR (amino acid response) pathway activation (100 nM HF/2 mM Pro/MEF; 10 nM HF/1 mM Pro/Murine CD4+ CD25- T cells). Shown to inhibit bFGF-induced neovascularization in a murine corneal angiogenesis model (5 mg/kg/day via food intake) and significantly reduce the severity of myelin antigen MOG33-55-induced autoimmune EAE/encephalomyelitis in mice (2 µg/mouse/day i.p.) in vivo. Unlike AA-AMP mimetics, HF does not compete against ATP for ProRS binding. ATP, in its ProRS bound state, actually helps stabilize HF via hydrogen bond interactions.
A cell-permeable racemic mixture of Halofuginone whose D-(+)/(2R, 3S) enantiomer serves as the active inhibitor against ProRS-mediated aminoacylation by simultaneously preventing ProRS-catalyzed Pro-AMP formation and the subsequent Pro transfer from Pro-AMP to tRNAPro. Proline, but not other NEAA, is able to reverse the inhibitory effect of HF in in vitro rabbit reticulocyte lysate translations (1 µM HF; 1 mM Pro) and prevent HF-induced cellular AAR pathway activation (100 nM HF/2 mM Pro/MEF; 10 nM HF/1 mM Pro/Murine CD4+ CD25- T cells). Shown to inhibit bFGF-induced neovascularization in a murine corneal angiogenesis model (5 mg/kg/d via food intake) and significantly reduce the severity of MOG33-55-induced EAE in mice (2 µg/mouse/d i.p.) in vivo. Unlike AA-AMP mimetics, HF does not compete against ATP for ProRS binding.
生化/生理作用
Primary Target
prolyl-tRNA synthetase
包装
Packaged under inert gas
警告
Toxicity: Standard Handling (A)
重悬
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 3 months at -20°C.
其他说明
Zhou, H., et al. 2013, Nature494, 121.
Keller, T.L., et al. 2012, Nat. Chem. Biol.12, 311.
Sundrud, M.S., et al. 2009, Science324, 1334.
Elkin, M., et al. 2000, FASEB J.14, 2477.
Elkin, M., et al. 1999, Clin. Cancer Res.5, 1982.
法律信息
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
基本信息
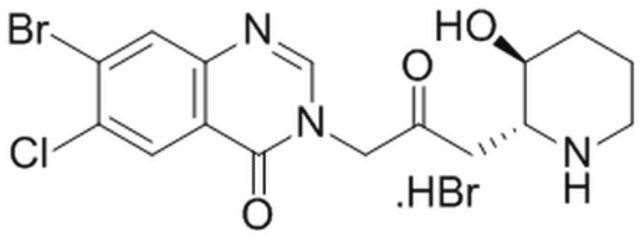
| 经验(实验)分子式 | C16H17BrClN3O3 · xHBr |
| 分子量 | 414.68 (free base basis) |
| MDL编号 | MFCD23843776 |
产品性质
| 质量水平 | 100 |
| 测定 | ≥98% (HPLC) |
| 形式 | powder |
| 效能 | 18 nM IC50 |
| manufacturer/tradename | Calbiochem® |
| 储存条件 | OK to freeze protect from light |
| 颜色 | white |
| 溶解性 | DMSO: 100 mg/mL |
| 储存温度 | 2-8℃ |
| InChI | 1S/C16H17BrClN3O3.BrH/c17-11-6-13-10(5-12(11)18)16(24)21(8-20-13)7-9(22)4-14-15(23)2-1-3-19-14;/h5-6,8,14-15,19,23H,1-4,7H2;1H/t14?,15-;/m1./s1 |
| InChI key | SJUWEPZBTXEUMU-NUNOUFIPSA-N |
安全信息
| 象形图 |   |
| 警示用语: | Danger |
| 危险声明 | H300 + H310 + H330 - H315 - H319 - H410 |
| 预防措施声明 | P262 - P273 - P280 - P302 + P352 + P310 - P304 + P340 + P310 - P305 + P351 + P338 |
| 危险分类 | Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Irrit. 2 |
| 储存分类代码 | 6.1A - Combustible, acute toxic Cat. 1 and 2 / very toxic hazardous materials |
| WGK | WGK 3 |
| 闪点(F) | Not applicable |
| 闪点(C) | Not applicable |