产品说明
一般描述
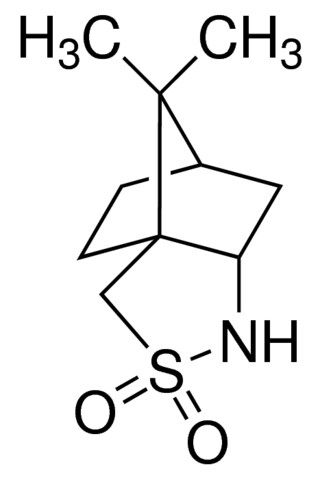
(1S,2R,4R)-(−)-2,10-Camphorsultam may be employed as a chiral probe for the optical resolution by HPLC and X-ray crystallographic determination of the absolute stereochemistry of carboxylic acids.
应用
(1S)-(−)-2,10-Camphorsultam may be used in the asymmetric synthesis of (S)- and (R)-N-Fmoc-S-trityl-α-methylcysteine. It may be used as proton source in the synthesis of chiral α,γ-substituted γ-butyrolactones.
包装
5 g in glass bottle
基本信息
| 经验(实验)分子式 | C10H17NO2S |
| 分子量 | 215.31 |
| Beilstein | 83811 |
| MDL编号 | MFCD00066271 |
| PubChem化学物质编号 | 24857980 |
| NACRES | NA.22 |
产品性质
| 质量水平 | 200 |
| 测定 | 98% |
| 旋光性 | [α]19/D −32°, c = 5 in chloroform |
| mp | 181-183 ℃ (lit.) |
| SMILES string | [H][C@@]12CC[C@]3(CS(=O)(=O)N[C@@H]3C1)C2(C)C |
| InChI | 1S/C10H17NO2S/c1-9(2)7-3-4-10(9)6-14(12,13)11-8(10)5-7/h7-8,11H,3-6H2,1-2H3/t7-,8-,10-/m1/s1 |
| InChI key | DPJYJNYYDJOJNO-NQMVMOMDSA-N |
安全信息
| 储存分类代码 | 13 - Non Combustible Solids |
| WGK | WGK 3 |
| 闪点(F) | Not applicable |
| 闪点(C) | Not applicable |
| 个人防护装备 | dust mask type N95 (US), Eyeshields, Gloves |