产品说明
一般描述
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
应用
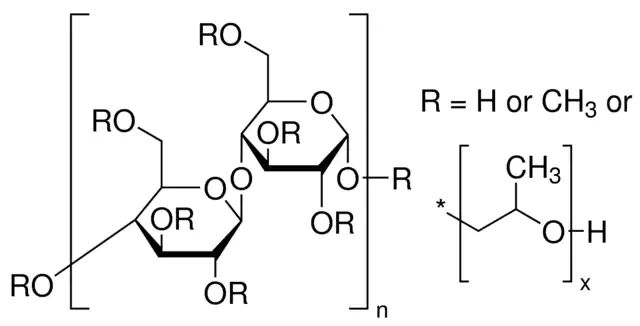
水性和非水性体系的增稠剂、耐油脂的透明薄膜、粘结剂、润滑剂、空间稳定剂和水助留剂。
特点和优势
可溶于水,加热时发生可逆胶凝作用,非离子性,不能与离子性物质形成复合物,具有表面活性和抗酶性。溶液具有假塑性。
分析说明
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
其他说明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
附注
To see an example of a Certificate of Analysis for this material enter LRAC0715 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
基本信息
| MDL编号 | MFCD00131360 |
| NACRES | NA.24 |
产品性质
| 质量水平 | 300 |
| 等级 | certified reference material pharmaceutical secondary standard |
| CofA | current certificate can be downloaded |
| 包装 | pack of 500 mg |
| application(s) | pharmaceutical |
| 格式 | neat |
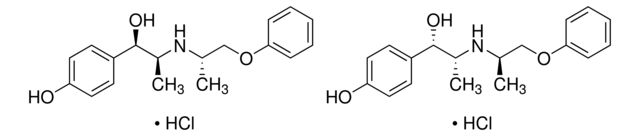
| 药典可追溯性 | traceable to USP 1330005 |
| 储存温度 | 2-30℃ |
安全信息
| 储存分类代码 | 13 - Non Combustible Solids |
| WGK | WGK 1 |
| 闪点(F) | Not applicable |
| 闪点(C) | Not applicable |