产品说明
一般描述
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
分析说明
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
其他说明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
基本信息
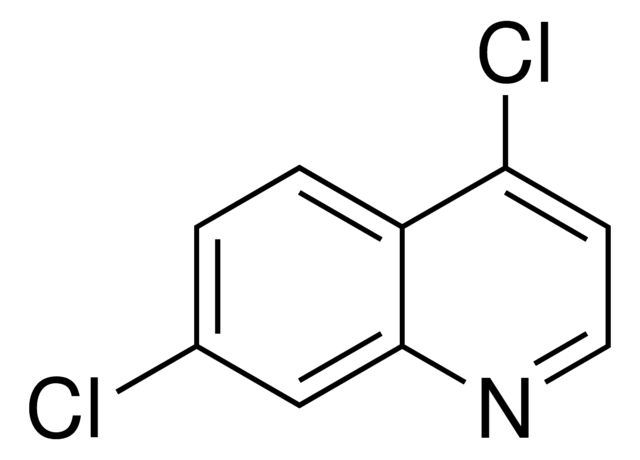
| 经验(实验)分子式 | C10H10ClNO |
| 分子量 | 195.65 |
产品性质
| 等级 | certified reference material pharmaceutical secondary standard |
| 形式 | powder |
| 包装 | pkg of 100 mg |
| 药典可追溯性 | traceable to PhEur B0200050 traceable to USP 1048222 |
| InChI | 1S/C10H10ClNO/c11-9-3-1-7(2-4-9)8-5-10(13)12-6-8/h1-4,8H,5-6H2,(H,12,13) |
| InChI key | OVRPDYOZYMCTAK-UHFFFAOYSA-N |
安全信息
| 象形图 |  |
| 警示用语: | Danger |
| 危险声明 | H301 |
| 预防措施声明 | P264 - P270 - P301 + P310 - P405 - P501 |
| 危险分类 | Acute Tox. 3 Oral |
| 储存分类代码 | 6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects |
| WGK | WGK 3 |
| 闪点(F) | Not applicable |
| 闪点(C) | Not applicable |