产品说明
一般描述
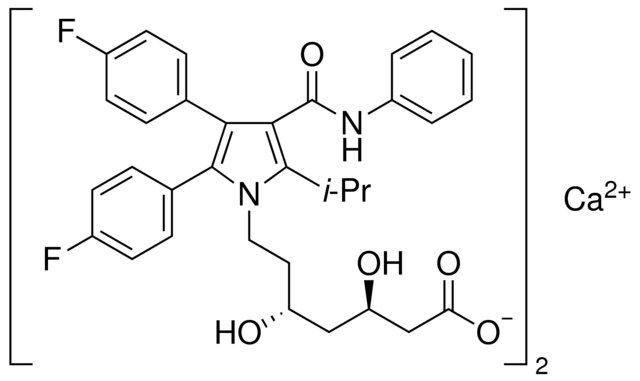
This material is an impurity of atorvastatin. Atorvastatin is a synthetic HMG-CoA reductase inhibitor which lowers plasma cholesterol levels by inhibiting endogenous cholesterol synthesis.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
应用
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Atorvastatin may be used as a pharmaceutical reference standard for the determination of the analyte in drug dosage forms by chromatography techniques.
分析说明
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
其他说明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
附注
To see an example of a Certificate of Analysis for this material enter LRAB7553 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
基本信息
| 经验(实验)分子式 | C66H66CaF4N4O10 |
| 分子量 | 1191.32 |
| NACRES | NA.24 |
产品性质
| 质量水平 | 300 |
| 等级 | certified reference material pharmaceutical secondary standard |
| CofA | current certificate can be downloaded |
| 包装 | pkg of 30 mg |
| application(s) | pharmaceutical |
| 格式 | neat |
| 药典可追溯性 | traceable to PhEur Y0001330 traceable to USP 1044549 |
| 储存温度 | -10 to -25℃ |
安全信息
| 储存分类代码 | 13 - Non Combustible Solids |
| WGK | WGK 3 |
| 闪点(F) | Not applicable |
| 闪点(C) | Not applicable |