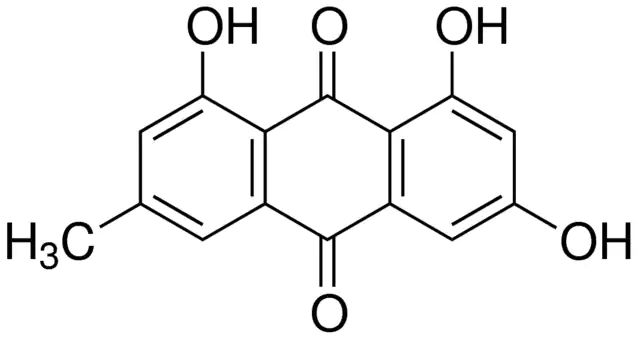
产品说明
一般描述
Certified pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to pharmacopeia primary standards
应用
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
分析说明
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
其他说明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
附注
To see an example of a Certificate of Analysis for this material enter LRAA2737 in the slot below. This is an example certificate only and may not be the lot that you receive.
基本信息
| NACRES | NA.24 |
产品性质
| 质量水平 | 300 |
| 等级 | certified reference material pharmaceutical secondary standard |
| CofA | current certificate can be downloaded |
| application(s) | pharmaceutical (small molecule) |
| 格式 | neat |
| 储存温度 | 2-30℃ |
安全信息
| 象形图 |  |
| 警示用语: | Warning |
| 危险声明 | H315 - H319 - H335 |
| 预防措施声明 | P261 - P305 + P351 + P338 |
| 危险分类 | Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3 |
| 靶器官 | Respiratory system |
| 储存分类代码 | 13 - Non Combustible Solids |
| WGK | WGK 3 |
| 闪点(F) | Not applicable |
| 闪点(C) | Not applicable |