产品说明
一般描述
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
生化/生理作用
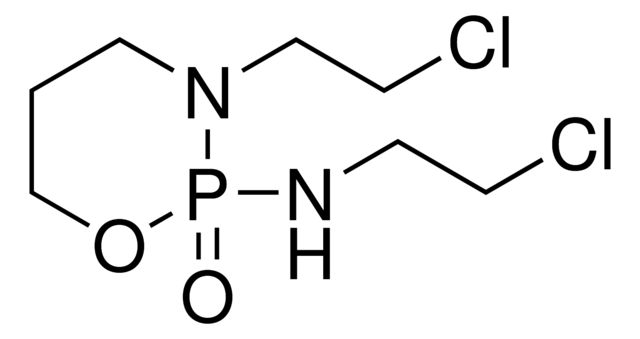
异环磷酰胺是一种氮芥类化合物,是环磷酰胺的结构异构体。 异环磷酰胺是一种前药,必须通过细胞色素P450转化为生物活性成分。 它在癌症化疗中可用作抗肿瘤药,但是异环磷酰胺比环磷酰胺更可能引起肾毒性。
分析说明
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
其他说明
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
附注
To see an example of a Certificate of Analysis for this material enter LRAA6959(changes by product) in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
基本信息
| 经验(实验)分子式 | C7H15Cl2N2O2P |
| 分子量 | 261.09 |
| MDL编号 | MFCD00057374 |
产品性质
| 等级 | certified reference material pharmaceutical secondary standard |
| CofA | current certificate can be downloaded |
| 包装 | pkg of 1 g |
| application(s) | pharmaceutical |
| 药典可追溯性 | traceable to BP BP759 traceable to USP 1336205 |
| 储存温度 | 2-8℃ |
| SMILES string | ClCCNP1(=O)OCCCN1CCCl |
| InChI | 1S/C7H15Cl2N2O2P/c8-2-4-10-14(12)11(6-3-9)5-1-7-13-14/h1-7H2,(H,10,12) |
| InChI key | HOMGKSMUEGBAAB-UHFFFAOYSA-N |
安全信息
| 象形图 |   |
| 警示用语: | Danger |
| 危险声明 | H301 - H319 - H340 - H350 - H360 |
| 预防措施声明 | P201 - P202 - P264 - P270 - P301 + P310 - P305 + P351 + P338 |
| 危险分类 | Acute Tox. 3 Oral - Carc. 1B - Eye Irrit. 2 - Muta. 1B - Repr. 1B |
| 储存分类代码 | 6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects |
| WGK | WGK 3 |
| 闪点(F) | Not applicable |
| 闪点(C) | Not applicable |