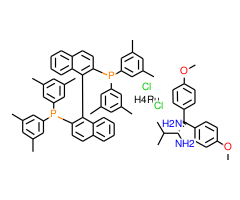
Technical Notes:
1.Highly active catalyst for hydrogenation of simple ketones giving high enantioselectivity when sterically unsymmetrical ketones such as acetophenone, heteroaryl ketones, benzophenones, cyclopropyl ketones, and cyclohexyl ketones are substrates. Ee's are enhanced with XyIBINAP relative to BINAP. The otherwise poorly bonded ketone is held in the transition state by hydrogen bonding to the protic bidentate amine.
2. Carbonyl groups are selectively reduced even when olefins exist in the s ame molecule.
3. In the presence of strong base, and catalyst, simple ketones, having substituents at the a-position, may be induced to undergo dynamic kinectic resolution during their hydrogenatio n toproduce , two chiral carbon centers in high yield.
References:
1. Angew Chem. Int. Ed.. 2001, 40, 40. (review article)
2.Org. Lott 2000, 2, 1749.
3.Org. Lott. 2000, 2, 659.
4.J. Am. Chem. Soc.. 1998. 120, 13529.
5.J. Am. Chem. Soc 2000, 122, 6510.