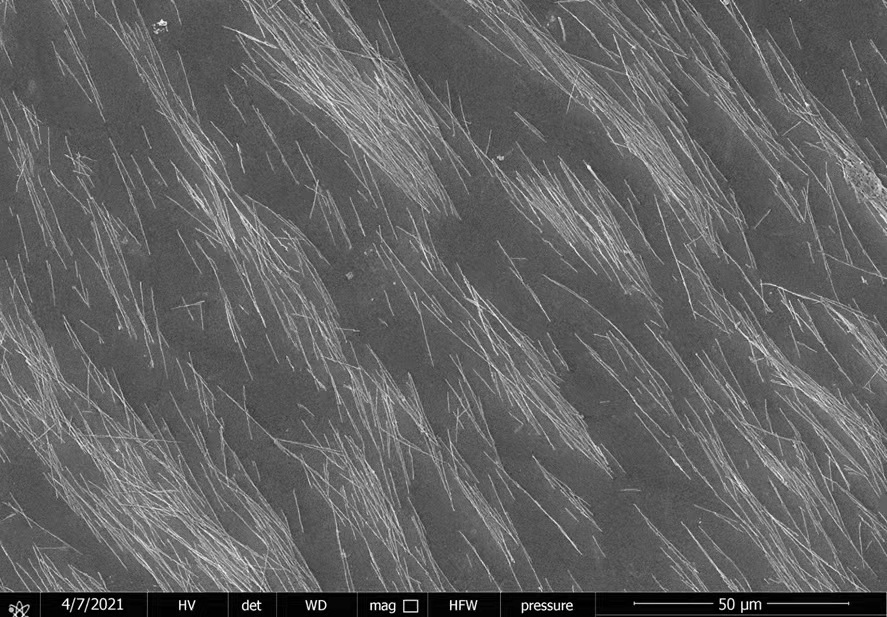
Technical Notes:
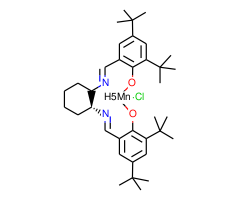
1.Catalyst for the conversion of olefins to chiral epoxides in high enantiomeric excess.
2.Pharmaceutically important, single enantiomer amino alcohols are efficiently produced from the corres ponding chiral epoxide by acid or base-catalyzed epoxide ring-opening reactions.
3.As ymmetric Kinetic res olution of secondary alcohols in water.
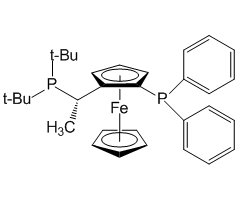
4.Enantios elective Reformatsky reaction with ketones.
References:
1.J. Org. Chem.. 1993, 58, 6939.
2.J. Am. Chem. Soc., 1994, 116, 6937
3.Encyclopedia of Reagents for Organic Synthesis, 1995, 7, 4585.
4.Angew. Chem. Int. Ed. Eng., 2003, 42, 1042.
5.Angew. Chem. Int. Ed. Eng., 2006, 45, 2951.