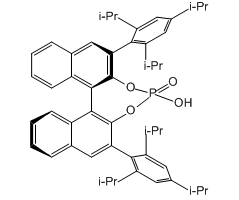
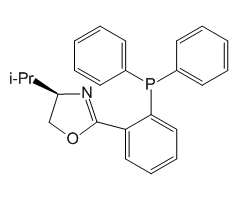
Technical Notes:
1.Reductive Amination: Catalyst for the organocatalytic asymmetric reductive amination of aldehydes.
2.Treating racemic a-branched alde hydes with p-anisidine and a Hantzsch ester in the presence of catalyst, TRIP, gave ß-branched secondary amines.
a-Allylation: Highly enantioselective Pd/chiral acid-catalyzed a-allylation of a-branched aldehydes with an allyl amine as the allylating species, that creates all-carbon quaternary stereogenic centers in high yields and enantioselectivities .
3.Hydrogenation: A achiral amine in combination with a catalytic amount of a chiral Brønsted acid can
accomplish an aldol addition-dehydration-conjugate reduction-reductive amination to provide potential
intermediates of pharmaceutically active compounds in good yields and excellent enantioselectivities.
4.Friedel-Crafts Reaction: The first enantioselective catalysis of the Friedel-Crafts reaction via activation of electron-rich multiple bonds by a chiral Brønsted acid.
5.Allylboration: A new high-yielding and highly enantioselective chiral Brønsted acid-catalyzed allylboration of aldehydes.
6.Aza-Darzens Reaction: Aza-Darzens reaction of ethyl diazoacetate with aldimines, derived from phenyl glyoxal, furnished cis-aziridine carboxylates with excellent enantioselectivities by means of a chiral phosphoric acid.
7.Intramolecular Aldol Condensation: Transformation applicable to a wide variety of substrates to give chiral cyclohexenones in high yields and with excellent enantios electivity.