Technical Notes:
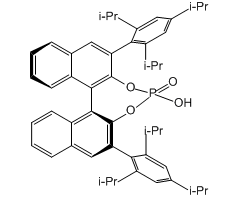
1.Pictet-Spengler Reaction: Catalyst for the asymmetric Pictet-Spengler reaction, where substituted
tryptamines are treated with an aldehyde in the presence of a catalytic amount of a chiral phos phoric acid.
2.Spiroketalization: The chiral catalyst can override the inherent preference for the formation of thermodynamic spiroketals, and highly selective formation of nonthermodynamic spiroketals could be achieved under the reaction conditions
3.oxa-Diels-Alder Cycloaddition: An asymmetric cascade annulation between 2-hydroxystyrenes and 2- alkynylbenaldehyes or 1 -(2-alkynylphenyl)ketones has been established with good to excellent
enantioselectivities, on the basis of an enantios elective oxa-Diels-Alder cycloaddition of in situ generated me tallo-isochromenylium intermediates, by cooperative binary catalysis of Pd(OAc)2 and (S)-TRIP
4.Hydrogenation: A 1 mol % loading of the chiral phosphoric acid catalyst converts aromatic and aliphatic
imines such as into the corresponding amines in high yields and enantioselectivities if treated with Hantzschdihydropyridine
5.Kinetic Resolution: An efficient and simple protocol for the kinetic resolution of secondary alcohols. The system is based on a combination of chiral Bronsted acid, DABCO, and acetyl chloride to gives various enantioenriched alcohols with selectivity factors up to 105.