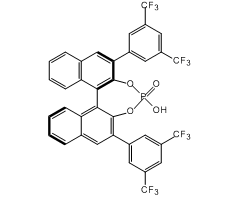
Technical Notes:
1.Reductive Amination Reaction: The first enantioselective organocatalytic reductive amination reaction has been accomplished.
2.Mannich Reaction: In the presence of a catalytic amt. of the phosphoric acid, anti-selective Mannich
reactions of cyclic ketones with a wide scope of aldimines were obtained.
3.The diastereoselectively switchable enantioselective trapping of protic carbamate ammonium ylides with imines is reported. The Rh2( )Ac)4 and chiral Brønsted acid cocatalyzed three-component Mannich-type reaction of a diazo compound, a carbamate, and an imine provides rapid and efficient access to both syn- and anti-a -substituted a, ß-diamino acid de rivatives .
4.Protonation: A catalytic asymmetric protonation of ketene dithioacetals is described. Various racemic a-aryl hyd rocoumarin derivatives are transformed into e nantioenriched dithioacetal- protected hydrocoumarins in
the presence of a chiral Brønsted acid catalyst.
5.Povarov Cyclization: Tetrahydroquinolines containing two quaternary stereogenic centers were
synthesized with excellent ee and dr via a four-component cyclization reaction catalyzed by a chiral
phosphoric acid.
6.Pictet-Spengler Reaction: ß-Carbolines could be synthesized with good enantioselectivity by the Pictet- Spengler reaction catalyzed by a chiral binol-derived Bronsted acid .
7.In the glycosylation of racemic alcohols with 1 using the chiral phosphoric acid as an activator, one
enantiomer of the racemic alcohol selectively reacts with 1 to give the corresponding glycoside with good to excellent a/ß-stereo- and diastereoselectivity in high yield.