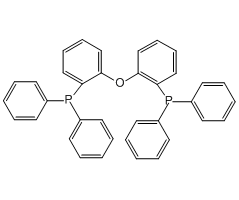
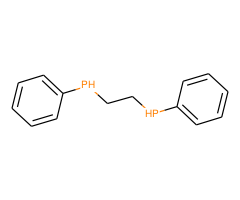
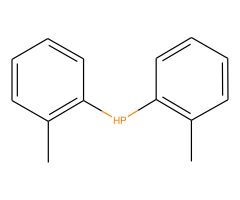
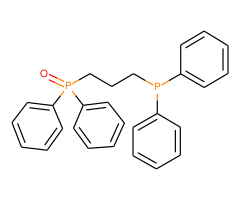
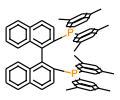
Technical Notes :
1. Biaryl bisphos phine ligand with narrow dihedral angle. The SEGPHOS® ligand has been applied
to a variety of metal catalyzed re actions. In many cases, yields and enantios electivities, exceed results obtained earlier using BINAP.1',2
2.As ruthenium complex, SEGPHOS® generally gives higher levels of chiral induction in as ymmetric hydrogenations of a,ß , and y-functionalized ketones. See ruthenium complexes 44-0096, 44-0518,44-0168.
3.Used in Rh-catalyzed transformations such as: (a) 1 ,4-addition of boronic acids to coumarins, (b) addition of titanium reagents to imines,' (c) cotrimerization of alkenes and acetylenes,"o (d)
double 2+2+2 cycloaddition,'' (e) indanone formation.
4. Used in Pd-catalyzed transformations such as: (a) cycloaddition of 1,6-enyne,' (b) arylative cyclization of allenyl aldehydes with boronic acids, s (c) synthesis of chromans .
5. Used in Cu-catalyzed transformations such as: (a) nitroso Diels-Alder,' (b) reductive aldol
condensation, (c) conjugate reduction of uns aturated sulfones,'5 and phophonates Iridium-catalyzed asymmetric hydrogenation of quinolines activated by chloroformates,
Iridium-catalyzed asymmetric transfer hydrog enation used in polyketide construction.
8.Rhodium-catalyzed as ymmetric hydroarylation of 3-pyrrolines.'
9. P alladium-catalyzed regio- and enantios elective dearomatization of pyrroles to 2H-pyrroles.19 10. 10.Rhodium- catalyzed as ymmetric s ynthesis of cyclopentanols 20
11Silver-catalyzed asymmetric Mannich-type reaction.