Technical Notes:
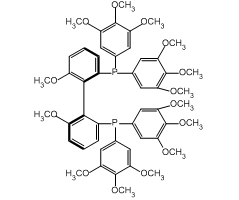
1. Biaryl bisphosphine ligand with narrow dihedral angle. The DTBM SEGPHOS® ligand, as the ruthenium complex, gives superior enantioselectivity and diastereoselectivity through dynamic kinetic resolution in the asymmetric hydrogenation of a-substituted-ß-ketoesters useful in the synthesis of carbapenum antibiotics.
2.With rhodium, preferential enantioselective hydrogenation of more reactive olefin of extended enone structure.
3.Rhodium catalyzed chemo-, regio, and entantioselective 2 + 2 + 2 cycloaddition of alkynes with isocyanates .
4.With copper, enantioselective cross Aldol-type reaction of acetonitrile.
5.With copper, enantioselective vinylsilane alkenylation of aldehydes.
6.Gold carbene mediated ste reoselective cyclopropanation of propargyl esters.
7.With copper, enantioselective 1 ,2-reduction of ketones, and 1 ,4-reduction of a a, ß-usaturatedesters
8.With copper, catalytic enantioselective Mannich-type reaction.
9.Enantioselective fluorination of b ß-keto esters, tert butoxycarbonyl lactones and lactmes with Sodeoka's Pd-aqua complex and a fluorinating reagent.
10.Rh-catalyzed intramolecular olefin or carbonyI hydroacylation.
11.Pd-catalyzed y-arylation of ß,y-unsaturated ketones .
12.Involved in numerous conjugate alkynylation, and ring-opening alkynylation of azabenzonorbornadienes.
13.Involved in asymmetric hydroamination of bicyclic alkenes/dienes,13a diamination of conjugated dienes, 13b and hydroalkoxylation/hydrosulfenylation of allenes.
14.Used in cycloaddition reactions such as 1,3- dipolar cycloaddition of azomethine ylides, and Au-catalyzed 2+2 cycoaddition of allenes.14b
15.Asymmetric conjugate addition of nitroalkanes to a,ß-usaturated thioamides .
16.Asymmetric synthesis of isothiazoles through Cu catalyzed conjugate addition of allyl cyanide to a,ß-usaturated thioamides.
17.Asymmetric Ag-catalyzed cycloadditions.
18.Rhodium-catalyzed c C bond cleavage to generate acyclic, asymmetric quaternary centers .
19Iridium-catalyzed intermolecular hydroamidation of olefins.
20.Palladium-Catalyzed Asymmetric Hydrogenation of a-Acyloxy-1 -arylethanones.