Technical Notes:
1. Michael Addition- Michael reaction of malonates to afforded Michael adducts with high yields and enantioselectivities (up to 95%, up to 93% ee).
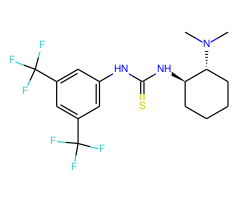
2.Synthesized a new class of bifunctional catalysts bearing a thiourea moiety and an amino group on a chiralscaffold.
3.A thiourea-catalyzed asymmetric Michael addition of activated methylene compounds to a,ß-unsaturated imides derived from 2-pyrrolidinone and 2-methoxybenzamide.
4.High enantioselectivities (up to 94 % ee) were attained in the Michael addition of a variety of a,ß -unsaturated imides (1) and malononitrile.
5.Alkylation- Primary aminothiourea derivatives catalyze enantioselective alkylation of a-arylpriopionaldehdyes with diarylbromomethane.
6.Living Ring-Opening Polymerization- A versatile, metal-free, organocatalytic approach to the living ring- opening polymerization of lactide.
7.Neber Reaction- The first enantioselective Neber reaction of ß-ketoxime sulfonates catalyzed by a
bifunctional thiourea.