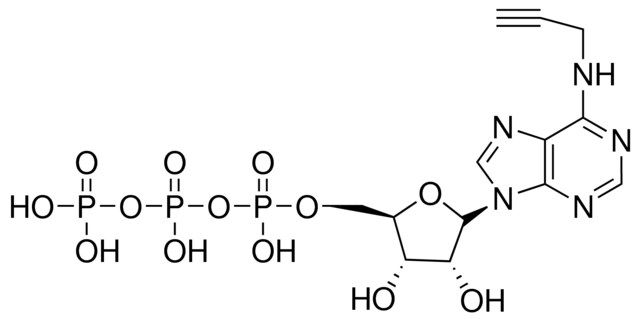
产品说明
应用
N6-Propargyl-ATP is suitable for in vitro AMPylation of proteins as well as in vitro polyadenylation of RNA. The resulting alkyne-functionalized protein or RNA can be processed using Cu(I)-catalyzed click chemistry to attach azide-labeled molecules. This gives options such as introduction of a biotin group for purification tasks, introduction of a fluorescent group for detection, or crosslinking to other azide-functionalized biomolecules.
包装
1 mg in glass bottle
法律信息
The purchase of this product for use in applications relating to copper catalyzed azide-alkyne cycloaddition chemistry ("Click Chemistry") includes a limited, nontransferable license to intellectual property owned by The Scripps Research Institute to use this product solely for internal non-commercial research activities and specifically excludes clinical, therapeutic, or diagnostic use in humans or animals and manufacturing purposes. Information regarding a license for commercial use in Click Chemistry may be obtained directly from The Scripps Research Institute, 10550 N. Torrey Pines Rd., La Jolla, CA 92037, or by contacting 858-784-8140 or click@scripps.edu.
基本信息
| 经验(实验)分子式 | C13H18N5O13P3 · xNa+ |
| 分子量 | 545.23 (free acid basis) |
| PubChem化学物质编号 | 329769102 |
| NACRES | NA.22 |
产品性质
| 质量水平 | 200 |
| 测定 | ≥95% (HPLC) |
| 形式 | solid |
| reaction suitability | reaction type: click chemistry |
| 溶解性 | 10 mM Tris-HCl, pH 7.5: soluble |
| 运输 | wet ice |
| 储存温度 | −20℃ |
| SMILES string | O[C@@H]([C@H]1O)[C@@H](COP(O)(OP(O)(OP(O)(O)=O)=O)=O)O[C@@H]1N2C=NC3=C2N=CN=C3NCC#C |
| InChI | 1S/C13H18N5O13P3/c1-2-3-14-11-8-12(16-5-15-11)18(6-17-8)13-10(20)9(19)7(29-13)4-28-33(24,25)31-34(26,27)30-32(21,22)23/h1,5-7,9-10,13,19-20H,3-4H2,(H,24,25)(H,26,27)(H,14,15,16)(H2,21,22,23)/t7-,9-,10-,13?/m1/s1 |
| InChI key | MUOIWXPACQCYQC-RJNFYWFKSA-N |
安全信息
| 储存分类代码 | 13 - Non Combustible Solids |
| WGK | WGK 3 |
| 闪点(F) | Not applicable |
| 闪点(C) | Not applicable |