产品说明
一般描述
A cell-permeable phenylsulfonylbenzimidazole compound that is shown to dock in the ligand-binding pocket of both HNF4α and HNF4γ via in silico structural analyses and antagonizes HNF4α DNA binding activity (by 93% after 10 µM overnight treatment of HepG2 cells), effectively inhibiting HNF4α-dependent cellular activities, including HNF4α mRNA transcription (by 62% in murine insulinoma MIN6 and 84% in human hepatoma HepG2 cultures after 5 h and 48 h 5 µM inhibitor treatment, respectively) and OTC (omithine transcarbamoylase) promoter transcription (by 85% & >95% in human HNF4α-transfected HepG2 & CV-1 cells, respectively; 48 hr 1 µM treatment). HNF4γ inhibition by BI6015 is also reported to indirectly lead to decreased binding of transactivators, E47 & PDX-1, to insulin promoter in T6PNE cells (48 h 5 µM treatment). Although BI6015 is found to exhibit cancer-selective cytotoxicity toward a panel of 58 human cancer cells and Hep3B-Luc (Effective conc. 1 to 10 µM), but not primary murine hepatocytes, it does cause hepatic steatosis both in vitro (primary murine hepatocytes; 5 µM for 3 days) and in mice in vivo (10 to 30 mg/kg/day for 5 days via i.p.) and is effectively metabolized by liver enzymes, limiting its in vivo efficacy in treating human Hep3B-derived liver tumor in mice. BI6015 also inhibits Human CYP450 2C19 and rat L-type calcium channel (by 94% and 83%, respectively, at 10 µM), but not PPARγ or a panel of 41 receptors/enzymes of human, mouse, and rat origin.
A cell-permeable phenylsulfonylbenzimidazole that is shown to dock in the ligand-binding pocket of both HNF4α and HNF4γ and antagonize HNF4α DNA binding activity in HepG2 cells (by 93%; 10 µM overnight), effectively inhibiting HNF4α-dependent cellular activities (Effective conc. 1 to 5 µM). HNF4γ inhibition by BI6015 can also lead to decreased insulin promoter binding by transactivators E47 & PDX-1 in T6PNE cells (5 µM 48 h). Although BI6015 is found to exhibit cancer-selective cytotoxicity toward a panel of 58 human cancer cells and Hep3B-Luc (Effective conc. 1 to 10 µM), but not primary murine hepatocytes, it does cause hepatic steatosis both in vitro and in mice in vivo, limiting its use in animal studies. BI6015 also inhibits Human CYP450 2C19 and rat L-type calcium channel (by 94% and 83%, respectively, at 10 µM), but not PPARγ or a panel of 41 receptors/enzymes of human, mouse, and rat origin.
包装
25 mg in Glass bottle
Packaged under inert gas
生化/生理作用
Cell permeable: yes
Reversible: yes
Primary Target
HNFα & γ
警告
Toxicity: Standard Handling (A)
其他说明
Kiselyuk, A., et al. 2012. Chem. Biol.19, 806.
法律信息
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
基本信息
| 经验(实验)分子式 | C15H13N3O4S |
| 分子量 | 331.35 |
| MDL编号 | MFCD04406654 |
产品性质
| 质量水平 | 100 |
| 测定 | ≥99% (HPLC) |
| 形式 | powder |
| manufacturer/tradename | Calbiochem® |
| 储存条件 | OK to freeze protect from light |
| 颜色 | beige |
| 溶解性 | DMSO: 10 mg/mL |
| 运输 | ambient |
| 储存温度 | 2-8℃ |
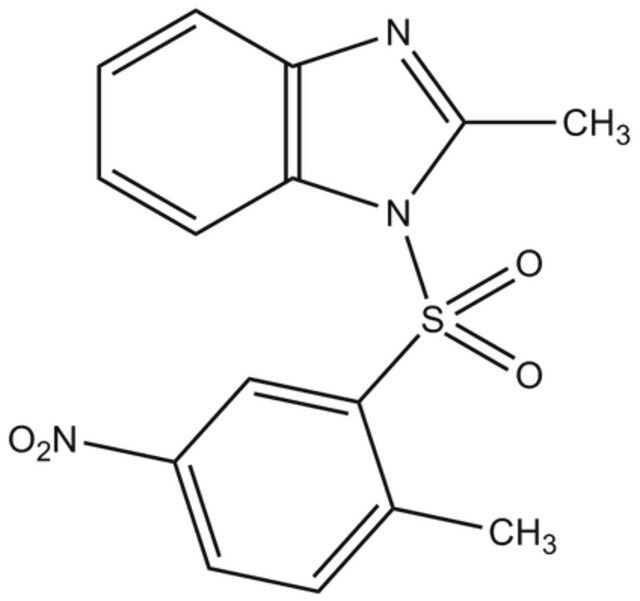
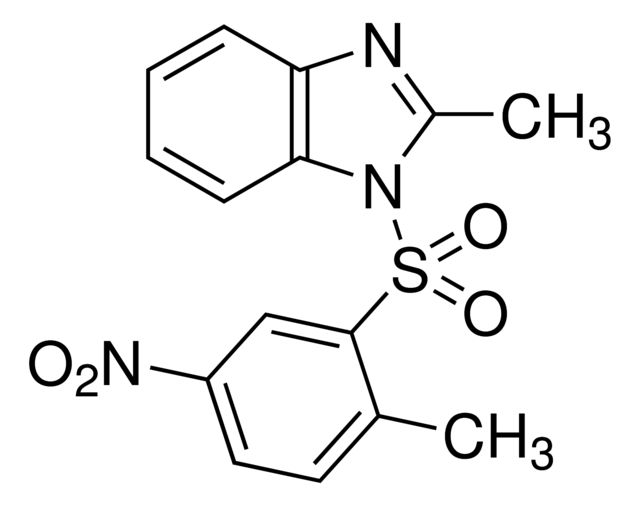
| SMILES string | CC1=NC2=C(N1S(C3=CC([N+]([O-])=O)=CC=C3C)(=O)=O)C=CC=C2 |
安全信息
| 储存分类代码 | 11 - Combustible Solids |
| WGK | WGK 2 |
| 闪点(F) | Not applicable |
| 闪点(C) | Not applicable |