产品介绍:
产品说明
应用
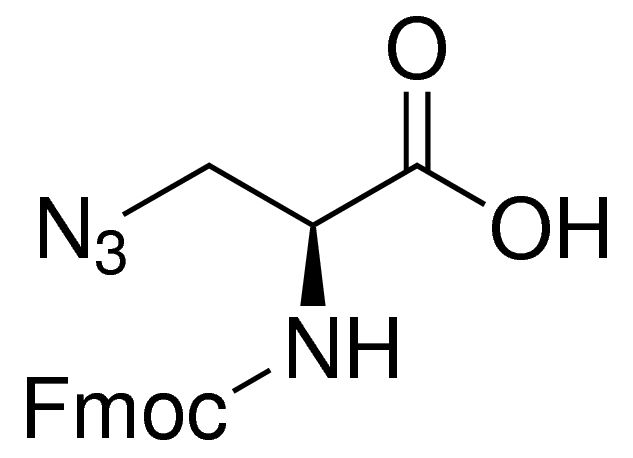
Fmoc-β-azido-Ala-OH chloride is a general N-terminal protected reagent used in the solid phase peptide synthesis. Azido group allows it to undergo copper-mediated click chemistry reactions.
It can be used in:
- Synthesis of α-N-acetylgalactosamine (α-GalNAc) linked antifreeze glycopeptides (AFGPs).
- Synthesis of stimuli-responsive multifunctional peptide gatekeepers for drug delivery applications.
- Synthesis of triazole-linked glycopeptides via Cu(I)-catalyzed 1,3-dipolar cycloaddition (CuAAC).
包装
250 mg in glass bottle
Bottomless glass bottle. Contents are inside inserted fused cone.
基本信息
| 经验(实验)分子式 | C18H16N4O4 |
| 分子量 | 352.34 |
| MDL编号 | MFCD11052919 |
| PubChem化学物质编号 | 329763258 |
| NACRES | NA.26 |
产品性质
| 质量水平 | 100 |
| 测定 | ≥98.0% (HPLC) |
| 旋光性 | [α]/D -10.0±1.0°, c = 1 in DMF |
| reaction suitability | reaction type: Fmoc solid-phase peptide synthesis reaction type: click chemistry |
| 杂质 | 15.3-16.5% nitrogen 60.1-62.5% carbon |
| application(s) | peptide synthesis |
| 官能团 | Fmoc |
| 储存温度 | 2-8℃ |
| SMILES string | OC(=O)[C@H](CN=[N+]=[N-])NC(=O)OCC1c2ccccc2-c3ccccc13 |
| InChI | 1S/C18H16N4O4/c19-22-20-9-16(17(23)24)21-18(25)26-10-15-13-7-3-1-5-11(13)12-6-2-4-8-14(12)15/h1-8,15-16H,9-10H2,(H,21,25)(H,23,24)/t16-/m0/s1 |
| InChI key | ZITYCUDVCWLHPG-INIZCTEOSA-N |
安全信息
| 储存分类代码 | 13 - Non Combustible Solids |
| WGK | WGK 3 |
| 闪点(F) | Not applicable |
| 闪点(C) | Not applicable |
| 个人防护装备 | dust mask type N95 (US), Eyeshields, Gloves |