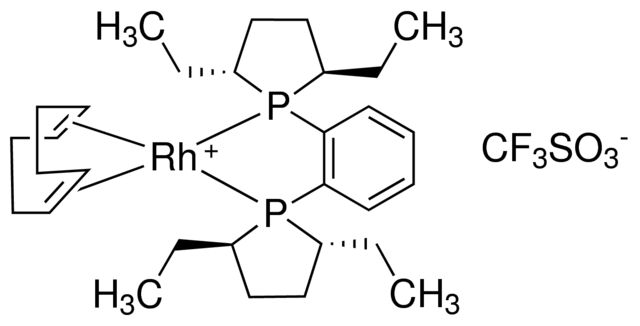
产品说明
应用
DuPhos and BPE Ligands: Highly Efficient Privileged Ligands
Catalyst for:
- Stereoselective synthesis of δ-amino acid derivatives via asymmetric hydrogenation of acetylaminopentenoic acid derivatives
- Stereoselective synthesis of manzacidins A and C via stereoselective hydrogenation
- Stereoselective synthesis of tetracyclic core of manzamine A via Rh-catalyzed asymmetric hydrogenation, diastereoselective Diels-Alder reaction, Eschenmoser-Tanabe fragmentation, Chang′s amide formation, and Hofmann rearrangement
- Asymmetric preparation of chiral Cbz-aminodifluorobutyric acid Me ester and its analogs
- Biphasic catalytic hydrogenations in ionic liquids with addition of water as a second solvent
- Preparation of cyclobutane-containing amino acids via asymmetric hydrogenations of cyclobutyl enamides
- Asymmetric preparation of both enantiomers of (dimethoxycoumaryl)alanine as suitable fluorescent peptide labels
包装
50, 250 mg in amber glass bottle
法律信息
与 Kanata Chemical Technologies Inc. 联合销售,仅供研究使用。这些化合物由 E.I. du Pont de Nemours and Company 授权制造和销售,此许可不包括利用这些化合物制备在制药领域销售的产品的权利。
基本信息
| 经验(实验)分子式 | C31H48F3O3P2RhS |
| 分子量 | 722.62 |
| MDL编号 | MFCD00269860 |
| PubChem化学物质编号 | 329761250 |
| NACRES | NA.22 |
产品性质
| 形式 | powder |
| SMILES string | [Rh+].[O-]S(=O)(=O)C(F)(F)F.C1CC=CCCC=C1.CC[C@@H]2CC[C@@H](CC)P2c3ccccc3P4[C@H](CC)CC[C@H]4CC |
| InChI | 1S/C22H36P2.C8H12.CHF3O3S.Rh/c1-5-17-13-14-18(6-2)23(17)21-11-9-10-12-22(21)24-19(7-3)15-16-20(24)8-4;1-2-4-6-8-7-5-3-1;2-1(3,4)8(5,6)7;/h9-12,17-20H,5-8,13-16H2,1-4H3;1-2,7-8H,3-6H2;(H,5,6,7);/q;;;+1/p-1/b;2-1-,8-7-;;/t17-,18-,19-,20-;;;/m1.../s1 |
| InChI key | HZLILTNLWVOBFS-KYOOHHHUSA-M |
安全信息
| 象形图 |  |
| 警示用语: | Warning |
| 危险声明 | H315 - H319 - H335 |
| 预防措施声明 | P261 - P264 - P271 - P280 - P302 + P352 - P305 + P351 + P338 |
| 危险分类 | Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3 |
| 靶器官 | Respiratory system |
| 储存分类代码 | 11 - Combustible Solids |
| WGK | WGK 3 |
| 闪点(F) | Not applicable |
| 闪点(C) | Not applicable |
| 个人防护装备 | dust mask type N95 (US), Eyeshields, Gloves |