产品说明
应用
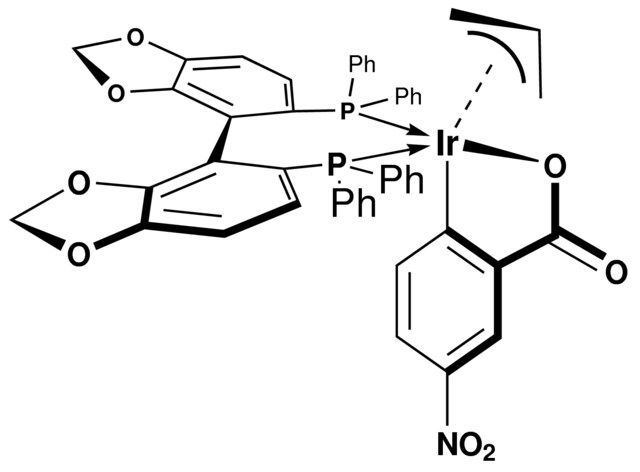
Catalyst developed by the Krische Group to affect the asymmetric carbonyl tert-prenylation (and allylation) from the alcohol or aldehyde oxidation level. Critical to the Krische group′s approach to Bryostatin 7, this catalyst is highly relevant for polyketide synthesis.
Total Synthesis of Bryostatin 7 via C–C Bond-Forming Hydrogenation
其他说明
Please note that sigma-aldrich provides this product to early discovery researchers as part of a collection of unique chemicals. sigma-aldrich does not collect analytical data for this product. Buyer assumes responsibility to confirm product identity and/or purity. All sales are final.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY; (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE; OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY; WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
基本信息
| 经验(实验)分子式 | C48H36IrNO8P2 |
| 分子量 | 1008.97 |
| MDL编号 | MFCD27978282 |
| PubChem化学物质编号 | 329824335 |
产品性质
| 形式 | solid |
| reaction suitability | core: iridium reagent type: catalyst |
| SMILES string | [C][C][C].O=C(O[Ir]1)C2=C1C=CC([N+]([O-])=O)=C2.P(C3=CC=CC=C3)(C4=CC=CC=C4)C5=CC=C6C(OCO6)=C5C7=C8C(OCO8)=CC=C7P(C9=CC=CC=C9)C%10=CC=CC=C%10 |
| InChI | 1S/C38H28O4P2.C7H4NO4.C3H5.Ir/c1-5-13-27(14-6-1)43(28-15-7-2-8-16-28)33-23-21-31-37(41-25-39-31)35(33)36-34(24-22-32-38(36)42-26-40-32)44(29-17-9-3-10-18-29)30-19-11-4-12-20-30;9-7(10)5-2-1-3-6(4-5)8(11)12;1-3-2;/h1-24H,25-26H2;1,3-4H,(H,9,10);3H,1-2H2;/q;;;+1/p-1 |
| InChI key | IQGKEOYRCUDYRW-UHFFFAOYSA-M |
安全信息
| 储存分类代码 | 13 - Non Combustible Solids |
| WGK | WGK 3 |
| 闪点(F) | Not applicable |
| 闪点(C) | Not applicable |