产品说明
一般描述
Device Configuration: 2 canisters
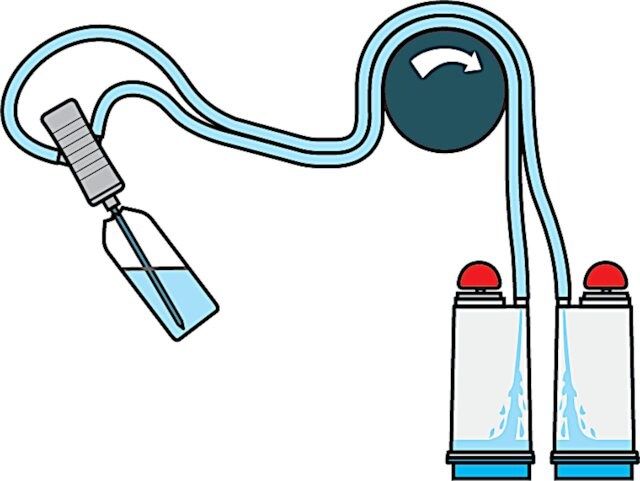
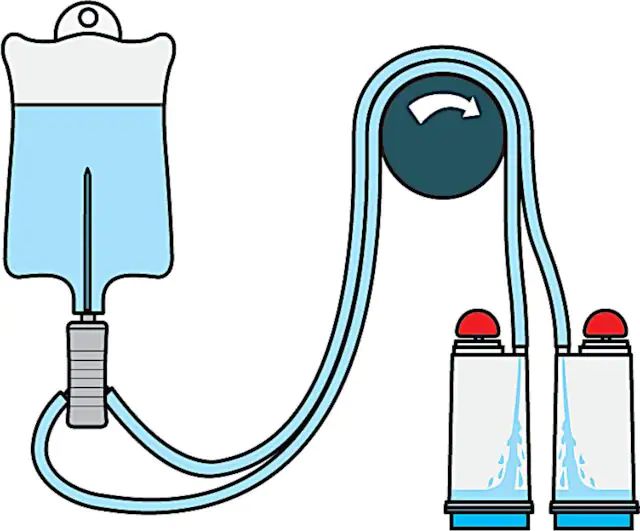
Steritest® NEO Device is a membrane filtration device for sterility testing of filterable pharmaceutical products. The device simplifies every aspect of testing, from handling to traceability. The closed system minimizes false positives and offers the highest levels of quality and reliability. This device ensures that pharmaceutical products are never exposed to the environment during the testing process. This test system offers an optimized and fully regulatory compliant testing process when used with the Steritest® Symbio pump, specific accessories and high-quality culture media and rinsing fluids. The device includes a single needle for easy access to ampoules and a separate vent needle. A single needle adapter enables easy access to collapsible bags. The canisters with blue base indicate mixed cellulose ester (MCE) membrane, which provides an optimal filtration flow rate for standard products.
应用
The Steritest® NEO Device is used for sterility testing of large volume parenterals and synthetic drugs without antimicrobial activity.
法律信息
MF-Millipore is a registered trademark of Merck KGaA, Darmstadt, Germany
STERITEST is a registered trademark of Merck KGaA, Darmstadt, Germany
基本信息
| eCl@ss | 32014001 |
| NACRES | NB.24 |
产品性质
| 质量水平 | 400 |
| 物料 | Nylon 66 adapter (for needle) PVC tubing (double lumen) mixed cellulose esters (MCE) membrane plain filter stainless steel (for needle) styrene-acrylonitrile (SAN) (for canister) |
| agency | EP (2.6.1) JP (4.06) USP 71 |
| 无菌性 | sterile; γ-irradiated |
| manufacturer/tradename | Steritest® |
| 包装 | pkg of 10 blisters per box, Single packed |
| 参数 | 3.1 bar max. inlet pressure (45 psi) at 25 ℃ 45 ℃ max. temp. |
| tubing L | 850 mm |
| 颜色 | blue Canister Base |
| 基质 | MF-Millipore® |
| 孔径 | 0.45 μm pore size |
| input | liquid sample type pharmaceutical(s) |
| 试验参数 | sample volume: 120 mL (graduation marks at 25, 50, 75 and 100 mL) |
| application(s) | pharmaceutical sterility testing |
| 运输 | ambient |
安全信息
| 储存分类代码 | 11 - Combustible Solids |
| WGK | WGK 2 |