产品介绍:
产品说明
一般描述
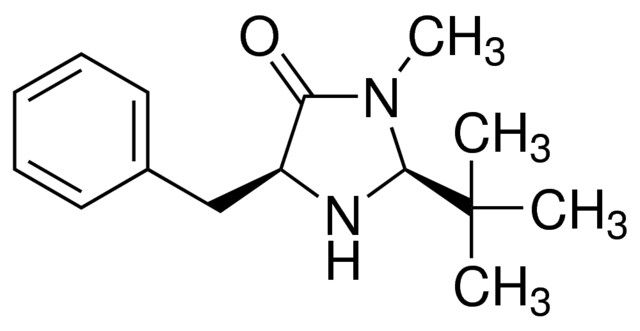
(2S,5S)-(-)-2-tert-Butyl-3-methyl-5-benzyl-4-imidazolidinone is a chiral imidazolidinone organocatalyst, developed by MacMillan and co-workers.
应用
(2S,5S)-(-)-2-tert-Butyl-3-methyl-5-benzyl-4-imidazolidinone is a second-generation MacMillan catalyst, which can be used as a chiral organocatalyst in:
- The chiral transformation reaction, including Friedel-Crafts and Mukaiyama-Michael reactions.
- The preparation of substituted spiroundecenetriones via asymmetric domino Knoevenagel/Diels-Alder reactions.
- The asymmetric synthesis of β-hydroxy aldehydes and their dimethylacetals via aldehyde-aldehyde aldol condensation reaction.
- The enantioselective α-fluorination of aldehydes using N-fluorobenzenesulfonamide as a fluorinating agent.
- The stereoselective preparation of (oxomethyl)oxabicyclo[3.2.1]octenones and tricyclic pyrroles via [4+3] cycloaddition of (trialkylsiloxy)pentadienals to furans.
Metal-free OrganoCatalyst technology for asymmetric catalysis. Catalyzes asymmetric indole alkylations, Friedel-Crafts alkylations, and a broad range of conjugate addition reactions in high enantiomeric excess.
包装
1 g in glass bottle
500 mg in glass insert
特点和优势
Advantages of MacMillan imidazolidinone organocatalysts:
- Superior enantiocontrol in numerous transformations
- High activities at low catalyst loadings
- Extraordinary functional group tolerance
法律信息
适用美国专利 6,369,243 和相关专利。仅供研究使用。
基本信息
| 经验(实验)分子式 | C15H22N2O |
| 分子量 | 246.35 |
| MDL编号 | MFCD03426982 |
| PubChem化学物质编号 | 24884185 |
| NACRES | NA.22 |
产品性质
| 质量水平 | 100 |
| 测定 | 97% |
| mp | 93-100 ℃ (lit.) |
| SMILES string | CN1[C@H](N[C@@H](Cc2ccccc2)C1=O)C(C)(C)C |
| InChI | 1S/C15H22N2O/c1-15(2,3)14-16-12(13(18)17(14)4)10-11-8-6-5-7-9-11/h5-9,12,14,16H,10H2,1-4H3/t12-,14-/m0/s1 |
| InChI key | SKHPYKHVYFTIOI-JSGCOSHPSA-N |
安全信息
| 储存分类代码 | 13 - Non Combustible Solids |
| WGK | WGK 3 |
| 闪点(F) | Not applicable |
| 闪点(C) | Not applicable |
| 个人防护装备 | Eyeshields, Gloves, type N95 (US) |