产品说明
一般描述
We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Greener Chemistry. This product has been enhanced for catalytic efficiency. Click here for more information.
应用
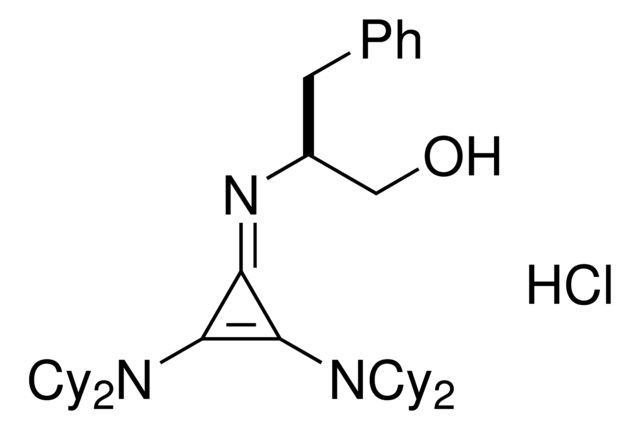
Chiral cyclopropenimines are a highly effective new class of enantioselective Brønsted base catalysts - the so-called “superbases” for enantioselective organocatalysis. Due to the prevalence of chemical reactions involving proton transfer as a key mechanistic event, Brønsted bases have become indispensable tools for the practice of organic synthetic chemistry, capable of catalyzing proton transfer reactions enantioselectively for the production of optically enriched products. Catalyst is stored as co-salt for stability. Conversion of the HCl salt to free catalyst requires a simple wash with aqueous base. This is one of a suite of Brønsted catalysts reported by Tristan Lambert and coworkers available through sigma-aldrich.
其他说明
Enantioselective Bronsted Base Catalysis with Chiral Cyclopropenimines
Cyclopropenimine-Catalyzed Enantioselective Mannich Reactions of tert-Butyl Glycinates with N-Boc-Imines
Transition State Analysis of Enantioselective Bronsted Base Catalysis by Chiral Cyclopropenimines
Structure-activity relationship studies of cyclopropenimines as enantioselective Bronsted base catalysts
Asymmetric Bronsted Base-Catalyzed and -Directed [3+2] Cycloaddition of 2-Acyl Cycloheptatrienes with Azomethine Ylides
基本信息
| 经验(实验)分子式 | C36H55N3O · HCl |
| 分子量 | 582.30 |
产品性质
| 质量水平 | 100 |
| 测定 | ≥95% |
| 形式 | powder or solid |
| reaction suitability | reagent type: catalyst reaction type: Asymmetric synthesis |
| 环保替代产品特性 | Catalysis Learn more about the Principles of Green Chemistry. |
| 环保替代产品分类 | Aligned |
安全信息
| 储存分类代码 | 13 - Non Combustible Solids |
| WGK | WGK 3 |
| 闪点(F) | Not applicable |
| 闪点(C) | Not applicable |