产品介绍:
产品说明
一般描述
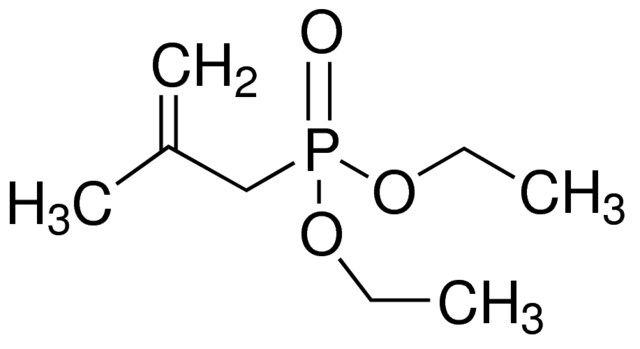
Diethyl (2-methylallyl)phosphonate is an organophosphorous compound. It participates in the synthesis of α-aminophosphonate derivatives and azaphosphones. The analgesic/antiinflammatory properties of these derivatives were evaluated.
应用
Diethyl (2-methylallyl)phosphonate can be used as a reagent in the Horner-Wadsworth-Emmons reaction to form conjugated carbon–carbon double bonds.
It can also be used as a reactant for:
- Enantioselective total synthesis of 10-isocyano-4-cadinene as antifouling agent.
- Regiospecific preparation of 4-oxo-2-alkenylphosphonates (OAP) via silylation followed by Friedel-Crafts acylation and isomerization. OAP can serve as building blocks for the construction of polyethylenic chains.
- The synthesis of azaphosphone as a potent analgesic/anti-inflammatory agents.
Reactant for:
- Enantioselective synthesis of 10-isocyano-4-cadinene and its stereoisomers with antifouling activity
- Preparation of 4-Oxo-2-alkenylphosphonates via silylation followed by regiospecific Friedel-Crafts acylation and isomerization
包装
1 g in glass bottle
基本信息
| 线性分子式 | (C2H5O)2POCH2C(=CH2)CH3 |
| 分子量 | 192.19 |
| MDL编号 | MFCD05664300 |
| PubChem化学物质编号 | 24881330 |
| NACRES | NA.22 |
产品性质
| 质量水平 | 100 |
| 测定 | 97% |
| reaction suitability | reaction type: C-C Bond Formation |
| 折射率 | n |
| bp | 62 ℃/0.1 mmHg (lit.) |
| 密度 | 1.013 g/mL at 25 ℃ (lit.) |
| SMILES string | CCOP(=O)(CC(C)=C)OCC |
| InChI | 1S/C8H17O3P/c1-5-10-12(9,11-6-2)7-8(3)4/h3,5-7H2,1-2,4H3 |
| InChI key | QOZGSMHGXZMADD-UHFFFAOYSA-N |
安全信息
| 象形图 |  |
| 警示用语: | Warning |
| 危险声明 | H319 |
| 预防措施声明 | P305 + P351 + P338 |
| 危险分类 | Eye Irrit. 2 |
| 储存分类代码 | 10 - Combustible liquids |
| WGK | WGK 3 |
| 闪点(F) | 230.0 °F - closed cup |
| 闪点(C) | > 110 ℃ - closed cup |
| 个人防护装备 | Eyeshields, Gloves, type ABEK (EN14387) respirator filter |